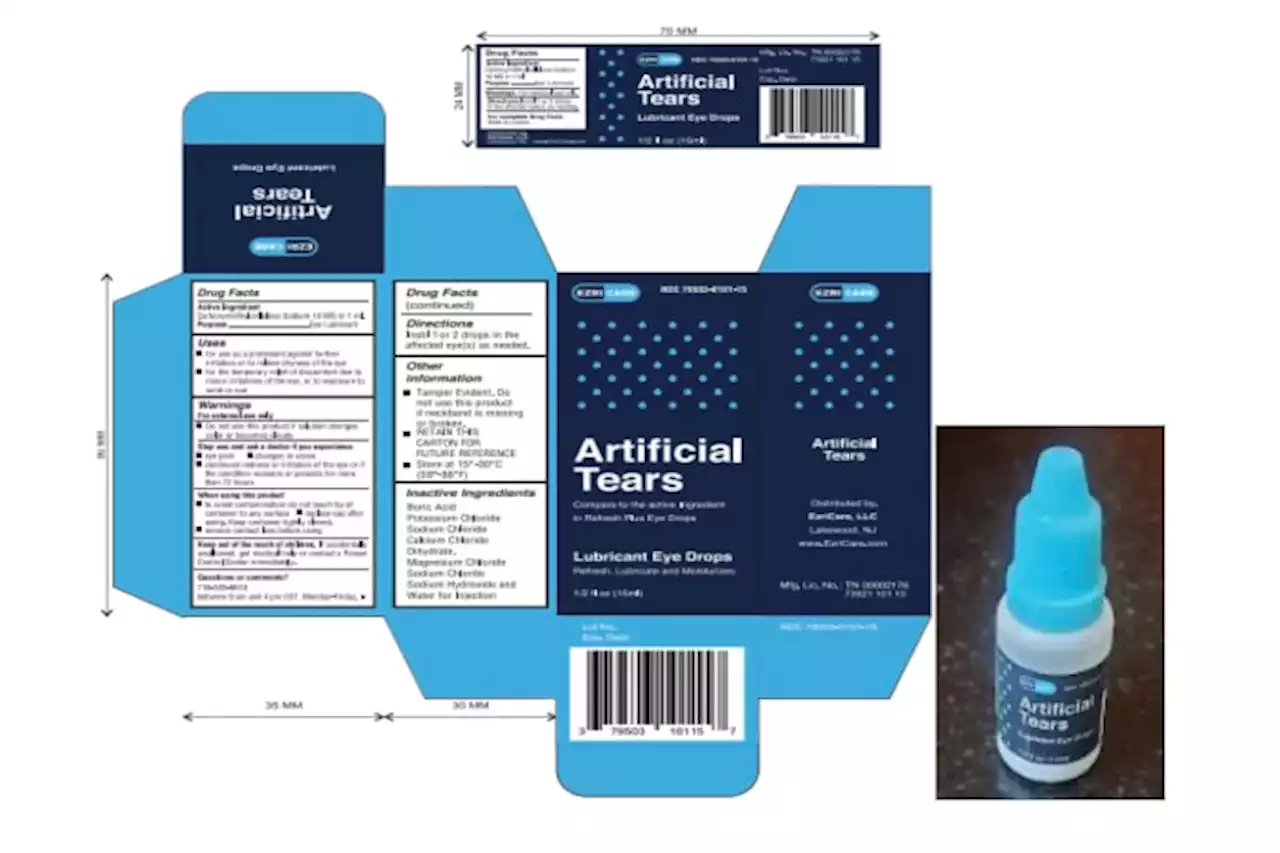
The Food and Drug Administration says it found a host of problems at an Indian manufacturing plant recently linked to contaminated eyedrops.
is considered particularly worrisome because the bacteria driving it is resistant to standard antibiotics.
The report has the agency’s preliminary findings and is likely to be followed by a formal report and a warning letter to the company. An FDA spokesman said the inspection indicates that the company’s products “may be in violation of FDA’s requirements.” The FDA is responsible for assuring the safety of foreign products shipped to the U.S., though it has long struggled to keep pace with international pharmaceutical supply chains that increasingly begin in India and China.
Global Pharma has said little publicly about its recent recalls, instead referring questions to the U.S. companies that sold the products.
Singapore Latest News, Singapore Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 Explainer: After COVID and drug shortages, EU revamps drug lawsThe EU is revamping laws governing the 136 billion euro ($148 billion) pharmaceuticals industry aimed at reviving investment and boosting access to affordable drugs at a time when health budgets have been drained by the costs of treating COVID-19.
Explainer: After COVID and drug shortages, EU revamps drug lawsThe EU is revamping laws governing the 136 billion euro ($148 billion) pharmaceuticals industry aimed at reviving investment and boosting access to affordable drugs at a time when health budgets have been drained by the costs of treating COVID-19.
Read more »
 Where exactly each dollar you spend on food goes: farmers get 14 cents and 4 cents pays for packagingA new report examines what each food dollar pays for, from food processing to packaging.
Where exactly each dollar you spend on food goes: farmers get 14 cents and 4 cents pays for packagingA new report examines what each food dollar pays for, from food processing to packaging.
Read more »
 Eyedrops maker couldn't ensure factory was sterile, FDA saysThe Food and Drug Administration says it found a host of problems at an Indian manufacturing plant recently linked to contaminated eyedrops
Eyedrops maker couldn't ensure factory was sterile, FDA saysThe Food and Drug Administration says it found a host of problems at an Indian manufacturing plant recently linked to contaminated eyedrops
Read more »
 Eyedrops maker couldn't ensure factory was sterile, FDA saysThe Food and Drug Administration says it found a host of problems at an Indian manufacturing plant recently linked to contaminated eyedrops.
Eyedrops maker couldn't ensure factory was sterile, FDA saysThe Food and Drug Administration says it found a host of problems at an Indian manufacturing plant recently linked to contaminated eyedrops.
Read more »
 Eyedrop recall: FDA finds sterilization issues at Global Health Pharma facilityThe U.S. Food and Drug Administration (FDA) found several issues at Global Pharma Healthcare's facility in India where it manufactured the recently recalled eye drops.
Eyedrop recall: FDA finds sterilization issues at Global Health Pharma facilityThe U.S. Food and Drug Administration (FDA) found several issues at Global Pharma Healthcare's facility in India where it manufactured the recently recalled eye drops.
Read more »
 Narcan to be available over the counter soon, though questions remain about costShari Conner, with the group Change 4 the Kenai, says keeping Narcan on hand is like making sure you have a fire extinguisher nearby.
Narcan to be available over the counter soon, though questions remain about costShari Conner, with the group Change 4 the Kenai, says keeping Narcan on hand is like making sure you have a fire extinguisher nearby.
Read more »