The FDA says it found a host of problems at an Indian manufacturing plant recently linked to contaminated eyedrops.
WASHINGTON — The manufacturer of eyedrops recently linked to deaths and injuries lacked measures to assure sterility at its factory in India, according to U.S. health inspectors.
The plant in India's southern Tamil Nadu state produced eyedrops that have been linked to 68 bacterial infections in the U.S., including three deaths and eight cases of vision loss. Four people have had their eyeballs surgically removed due to infection. The drops were recalled in February by two U.S. distributors, EzriCare and Delsam Pharma.
The report has the agency's preliminary findings and is likely to be followed by a formal report and a warning letter to the company. An FDA spokesman said the inspection indicates that the company's products "may be in violation of FDA's requirements." FDA inspectors cited worrisome sanitary conditions at the Global Pharma plant, noting that its floors, walls and ceilings were not "easily cleanable." At one point during the visit, an FDA inspector noted "none of the equipment on the filling machine was wrapped or covered." The inspector also noted the company didn't have rigorous procedures for ensuring bottles were fully sealed.
Singapore Latest News, Singapore Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 Eyedrops maker couldn't ensure factory was sterile, FDA saysThe Food and Drug Administration says it found a host of problems at an Indian manufacturing plant recently linked to contaminated eyedrops
Eyedrops maker couldn't ensure factory was sterile, FDA saysThe Food and Drug Administration says it found a host of problems at an Indian manufacturing plant recently linked to contaminated eyedrops
Read more »
 Eyedrops maker couldn't ensure factory was sterile, FDA saysThe Food and Drug Administration says it found a host of problems at an Indian manufacturing plant recently linked to contaminated eyedrops. FDA inspectors found about a dozen problems with how Global Pharma Healthcare made and tested the drops. The FDA released its preliminary inspection report Monday. Recalled eyedrops made at the plant have been linked to 68 bacterial infections in the U.S., including three deaths and eight cases of vision loss. Four people have had to have their eyeballs surgically removed due to infection. The outbreak is considered particularly worrisome because the bacteria driving it is resistant to standard antibiotics.
Eyedrops maker couldn't ensure factory was sterile, FDA saysThe Food and Drug Administration says it found a host of problems at an Indian manufacturing plant recently linked to contaminated eyedrops. FDA inspectors found about a dozen problems with how Global Pharma Healthcare made and tested the drops. The FDA released its preliminary inspection report Monday. Recalled eyedrops made at the plant have been linked to 68 bacterial infections in the U.S., including three deaths and eight cases of vision loss. Four people have had to have their eyeballs surgically removed due to infection. The outbreak is considered particularly worrisome because the bacteria driving it is resistant to standard antibiotics.
Read more »
 Maker of eyedrops linked to deadly infections couldn’t ensure factory was sterile, FDA saysThe manufacturer of eyedrops recently linked to deaths and injuries lacked measures to assure sterility at its factory in India, according to U.S. health inspectors.
Maker of eyedrops linked to deadly infections couldn’t ensure factory was sterile, FDA saysThe manufacturer of eyedrops recently linked to deaths and injuries lacked measures to assure sterility at its factory in India, according to U.S. health inspectors.
Read more »
 Eyedrops maker couldn't ensure factory was sterile, FDA saysThe manufacturer of eyedrops recently linked to deaths and injuries lacked measures to assure sterility at its factory in India, according to U.S. health inspectors. Food and Drug Administration officials uncovered about a dozen problems with how Global Pharma Healthcare made and tested its eyedrops during an inspection from late February through early March. The FDA released its preliminary inspection report Monday.
Eyedrops maker couldn't ensure factory was sterile, FDA saysThe manufacturer of eyedrops recently linked to deaths and injuries lacked measures to assure sterility at its factory in India, according to U.S. health inspectors. Food and Drug Administration officials uncovered about a dozen problems with how Global Pharma Healthcare made and tested its eyedrops during an inspection from late February through early March. The FDA released its preliminary inspection report Monday.
Read more »
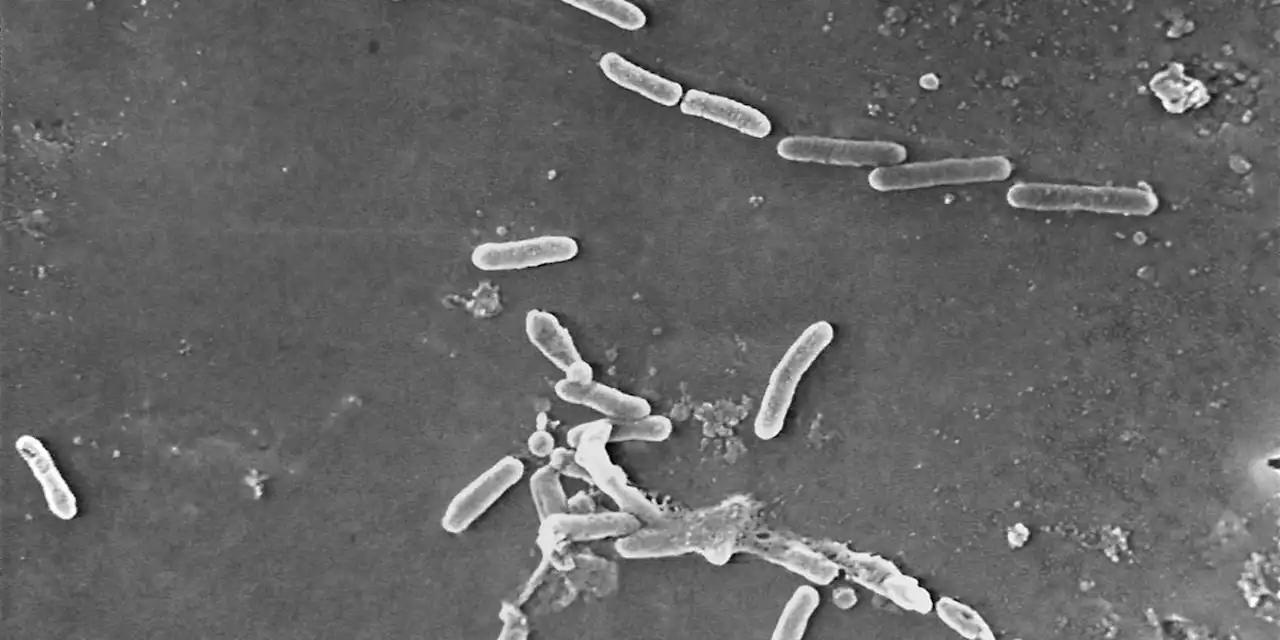 Eyedrops maker couldn’t ensure factory was sterile, FDA saysIn particular, the inspectors found that the plant had used “a deficient manufacturing process” between December 2020 and April 2022 for products that were later shipped to the U.S.
Eyedrops maker couldn’t ensure factory was sterile, FDA saysIn particular, the inspectors found that the plant had used “a deficient manufacturing process” between December 2020 and April 2022 for products that were later shipped to the U.S.
Read more »
 Eyedrops maker couldn't ensure factory was sterile, FDA saysThe Food and Drug Administration says it found a host of problems at an Indian manufacturing plant recently linked to contaminated eyedrops.
Eyedrops maker couldn't ensure factory was sterile, FDA saysThe Food and Drug Administration says it found a host of problems at an Indian manufacturing plant recently linked to contaminated eyedrops.
Read more »
