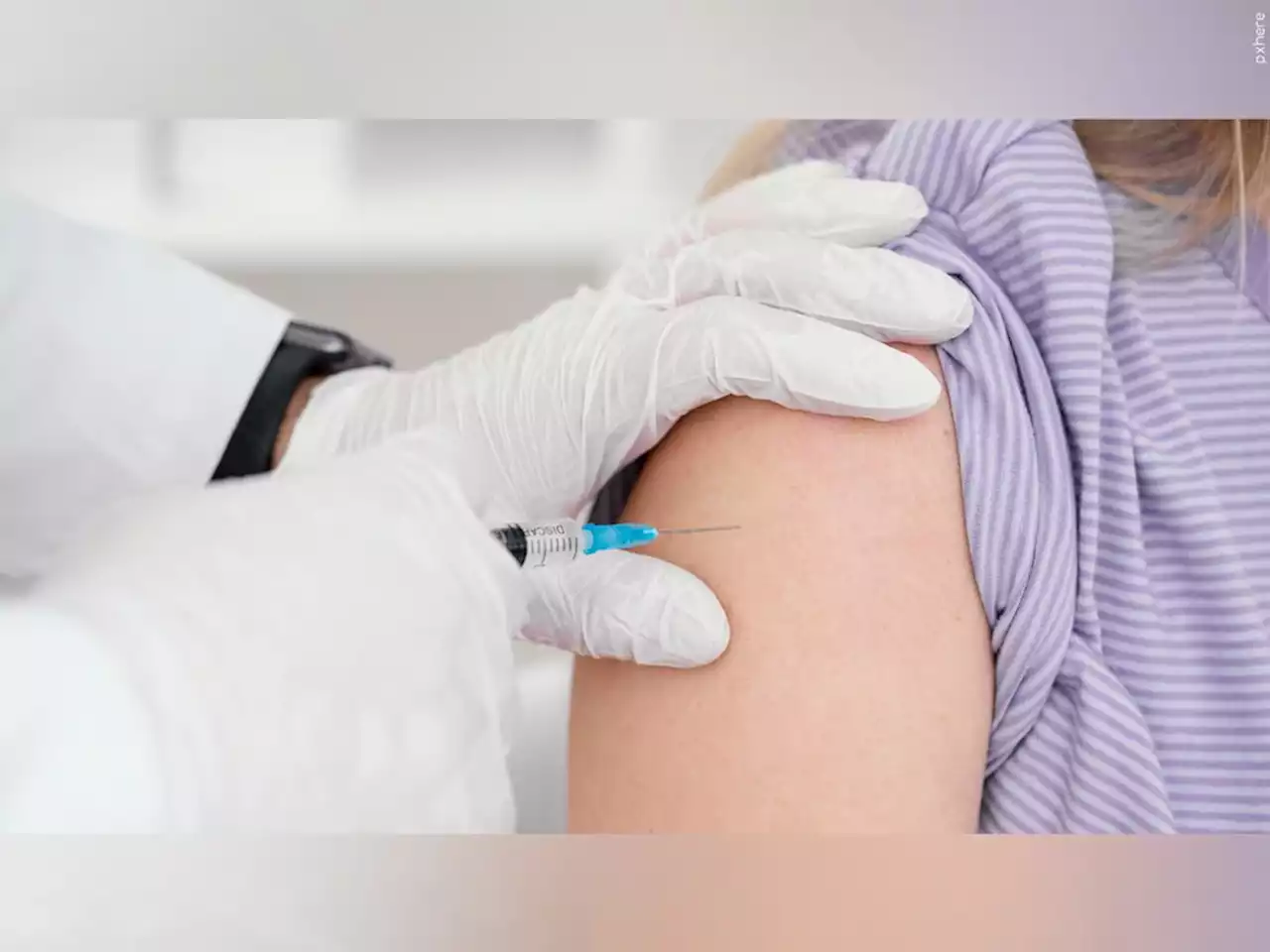
Vaccinations could begin as early as Monday or Tuesday.
WASHINGTON — U.S. regulators on Friday authorized the first COVID-19 shots for infants and preschoolers, paving the way for vaccinations to begin next week.for the shots from Moderna and Pfizer. That means U.S. kids under 5 — roughly 18 million youngsters — are eligible for the shots, about 1 1/2 years after the vaccines first became available in the U.S. for adults, who have been hit the hardest during the pandemic.
There’s one step left: The Centers for Disease Control and Prevention recommends how to use vaccines and its vaccine advisers are set to discuss the shots for the youngest kids Friday and vote on Saturday. A final signoff would come from CDC Director Dr. Rochelle Walensky. “So I actually think we need to protect young children, as well as protect everyone with the vaccine and especially protect elders,” she said.
“As we have seen with older age groups, we expect that the vaccines for younger children will provide protection from the most severe outcomes of COVID-19, such as hospitalization and death,” FDA Commissioner Robert Califf said in a statement.Pfizer’s vaccine for kids younger than 5 is one-tenth of the adult dose. Three shots are needed: the first two given three weeks apart and the last at least two months later.
Singapore Latest News, Singapore Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 FDA Advisers Review Moderna, Pfizer COVID-19 Shots for Kids as Young as 6 monthsU.S. government advisers met Wednesday to decide whether to endorse COVID-19 shots for babies, toddlers and preschoolers, moving the nation closer to vaccinations for all ages.
FDA Advisers Review Moderna, Pfizer COVID-19 Shots for Kids as Young as 6 monthsU.S. government advisers met Wednesday to decide whether to endorse COVID-19 shots for babies, toddlers and preschoolers, moving the nation closer to vaccinations for all ages.
Read more »
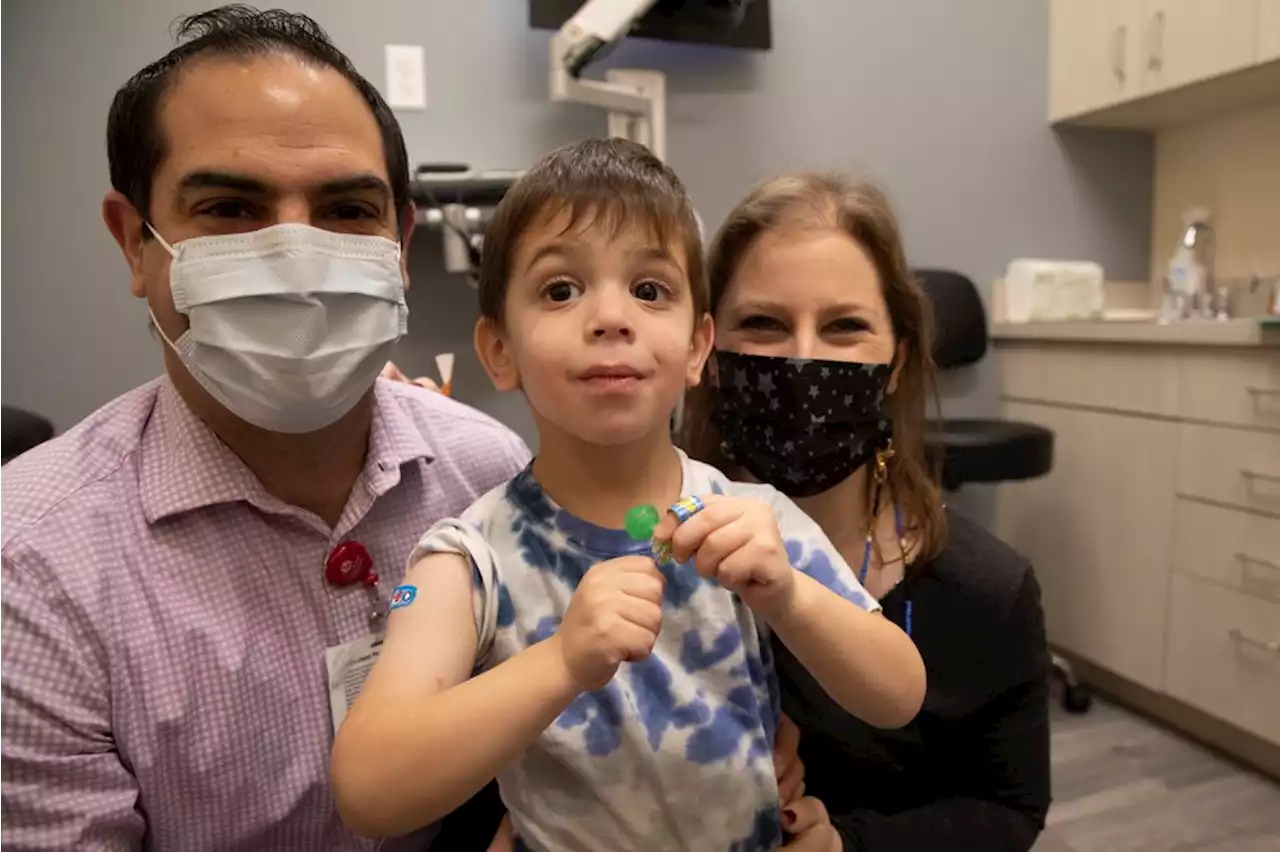 FDA advisers weigh COVID-19 shots for babies, young childrenU.S. government advisers met Wednesday to decide whether to endorse COVID-19 shots for the youngest children, moving the nation closer to vaccinations for all ages.
FDA advisers weigh COVID-19 shots for babies, young childrenU.S. government advisers met Wednesday to decide whether to endorse COVID-19 shots for the youngest children, moving the nation closer to vaccinations for all ages.
Read more »
 FDA Advisers Move Moderna's COVID-19 Shots Closer To Kids Under 5The outside experts voted unanimously that the benefits of Moderna’s shots outweigh any risks for children under 5.
FDA Advisers Move Moderna's COVID-19 Shots Closer To Kids Under 5The outside experts voted unanimously that the benefits of Moderna’s shots outweigh any risks for children under 5.
Read more »
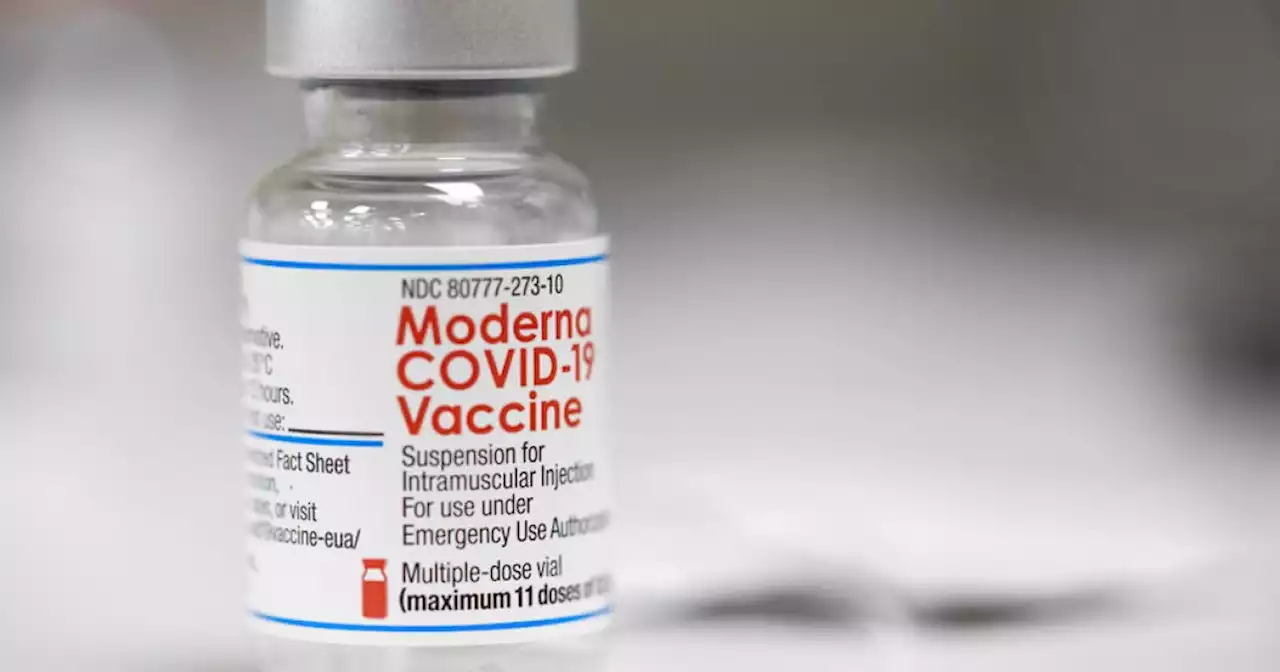 FDA panel says Pfizer, Moderna's COVID-19 shots for tots safe and effectiveUPDATE: FDA panel says Pfizer, Moderna's COVID-19 shots for kids under 5 are safe and effective
FDA panel says Pfizer, Moderna's COVID-19 shots for tots safe and effectiveUPDATE: FDA panel says Pfizer, Moderna's COVID-19 shots for kids under 5 are safe and effective
Read more »
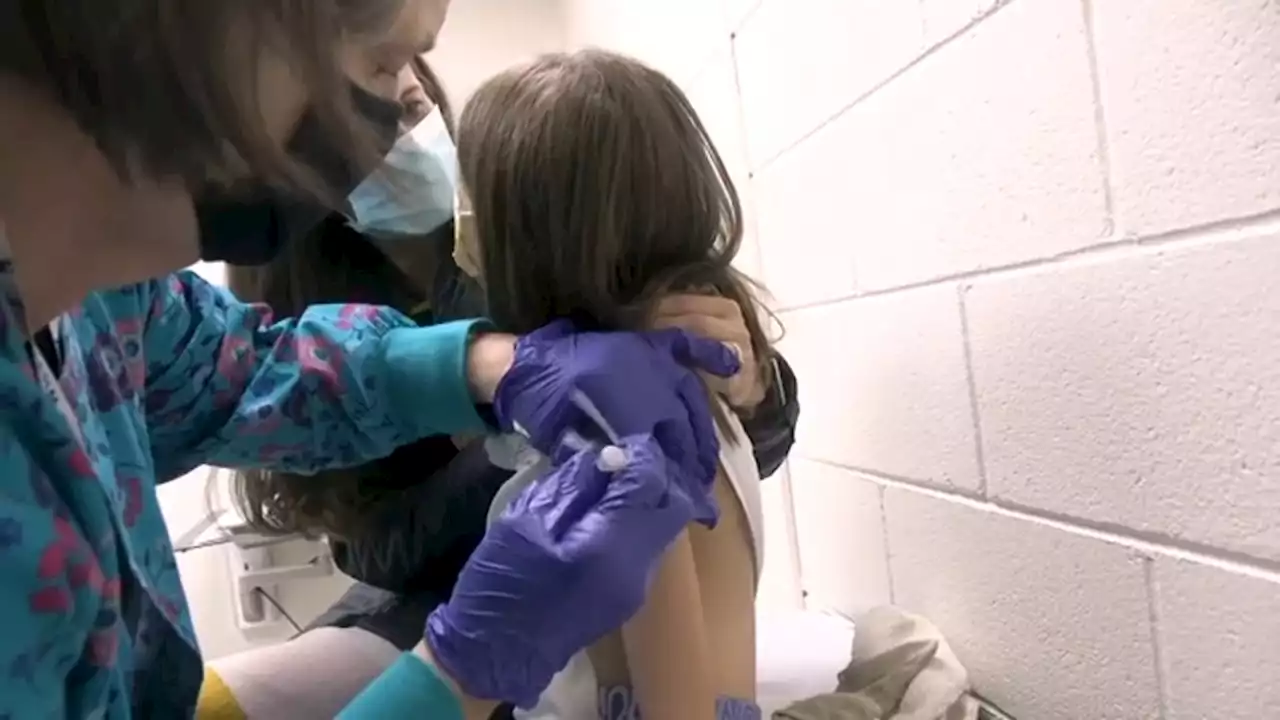 FDA advisory panel backs COVID-19 vaccine for younger kids, the last unprotected groupIf all the regulatory steps are cleared, shots should be available next week.
FDA advisory panel backs COVID-19 vaccine for younger kids, the last unprotected groupIf all the regulatory steps are cleared, shots should be available next week.
Read more »
 FDA advisers endorse emergency use of Moderna, Pfizer Covid-19 vaccine in babies, toddlersFDA advisers voted unanimously to endorse Moderna's Covid-19 vaccines for babies and toddlers. The FDA is expected to quickly authorize the vaccine for emergency use. A vote on Pfizer's vaccine is coming soon.
FDA advisers endorse emergency use of Moderna, Pfizer Covid-19 vaccine in babies, toddlersFDA advisers voted unanimously to endorse Moderna's Covid-19 vaccines for babies and toddlers. The FDA is expected to quickly authorize the vaccine for emergency use. A vote on Pfizer's vaccine is coming soon.
Read more »