The FDA said it plans to convene its outside panel of vaccine experts on June 8, 21 and 22 to review applications from Moderna and Pfizer for child vaccines. The dates are not final.
The FDA said it plans to convene its outside panel of vaccine experts on June 8, 21 and 22 to review applications from Moderna and Pfizer for child vaccines. The dates are not final and the FDA said it will provide additional details as each company completes their application.
On Thursday, Moderna submitted data to the FDA that it hopes will prove its two low-dose shots can protect children younger than 6. Moderna has filed FDA applications for older kids, but the FDA hasn’t ruled on them. It’s not clear if that data for older children will be considered at the June meetings.
Singapore Latest News, Singapore Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 FDA Panel to Discuss Novavax Covid Vaccine for Adults, Pfizer and Moderna Shots for Kids in JuneThe meetings are a sign that the vaccines are moving closer to a possible authorization.
FDA Panel to Discuss Novavax Covid Vaccine for Adults, Pfizer and Moderna Shots for Kids in JuneThe meetings are a sign that the vaccines are moving closer to a possible authorization.
Read more »
 Lawmakers scrutinize McKinsey's opioid, FDA consulting workLawmakers vowed to continue investigating consulting firm McKinsey after a hearing scrutinizing its work for the Food and Drug Administration even as it advised opioid makers on boosting sales.
Lawmakers scrutinize McKinsey's opioid, FDA consulting workLawmakers vowed to continue investigating consulting firm McKinsey after a hearing scrutinizing its work for the Food and Drug Administration even as it advised opioid makers on boosting sales.
Read more »
 Pfizer Asks FDA To Authorize Covid Booster For Kids 5-11As the omicron variant puts children at greater risk of hospitalization, public health officials have worked to make Covid-19 treatments more accessible to young people.
Pfizer Asks FDA To Authorize Covid Booster For Kids 5-11As the omicron variant puts children at greater risk of hospitalization, public health officials have worked to make Covid-19 treatments more accessible to young people.
Read more »
 Moderna to ask FDA to authorize Covid-19 vaccine for young kidsThe request comes a month after the drug company signaled its two-dose regimen generated immune protection in the youngest children comparable to young adults.
Moderna to ask FDA to authorize Covid-19 vaccine for young kidsThe request comes a month after the drug company signaled its two-dose regimen generated immune protection in the youngest children comparable to young adults.
Read more »
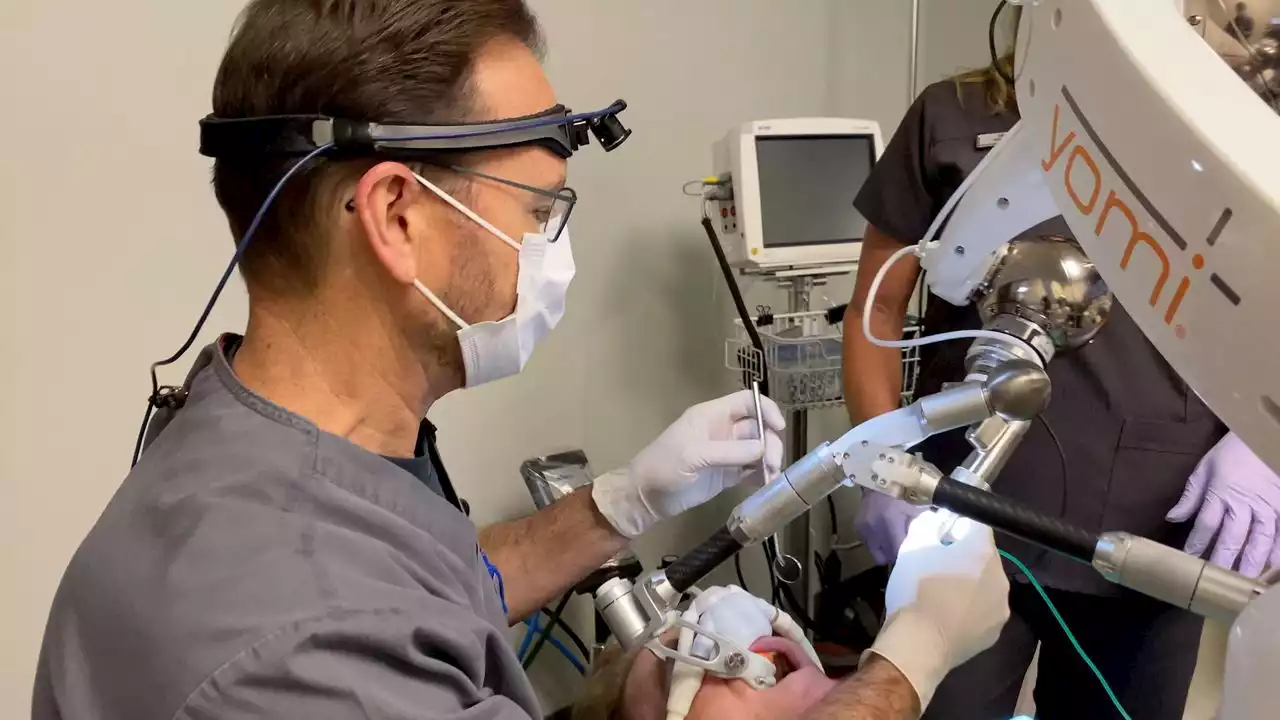 North Texas dentist uses first-ever FDA-cleared dental robotDr. J. Darrell Steele stays up-to-date with the latest technology in dentistry. When he learned about a robotic device cleared by the FDA as the first robot-assisted dental surgical system used for a single-tooth implant, he was interested.
North Texas dentist uses first-ever FDA-cleared dental robotDr. J. Darrell Steele stays up-to-date with the latest technology in dentistry. When he learned about a robotic device cleared by the FDA as the first robot-assisted dental surgical system used for a single-tooth implant, he was interested.
Read more »
 Lawmakers scrutinize McKinsey's opioid, FDA consulting workLawmakers vowed to continue investigating consulting firm McKinsey after a hearing scrutinizing its work for the Food and Drug Administration even as it advised opioid makers on boosting sales
Lawmakers scrutinize McKinsey's opioid, FDA consulting workLawmakers vowed to continue investigating consulting firm McKinsey after a hearing scrutinizing its work for the Food and Drug Administration even as it advised opioid makers on boosting sales
Read more »
