The FDA has attempted several times to get rid of menthol but faced pushback from Big Tobacco.
WASHINGTON -- The U.S. government on Thursday will lay out its long-awaited plan to ban menthol cigarettes and flavored cigars, which have taken a disproportionate toll on Black smokers and other minorities.
The FDA has attempted several times to get rid of menthol but faced pushback from Big Tobacco, members of Congress and competing political interests under both Democratic and Republican administrations. The agency's proposals on both cigarettes and cigars will only be initial drafts. FDA will take comments before issuing final rules, which then could face years of legal challenges from tobacco companies.
"Black folks die disproportionately of heart disease, lung cancer and stroke," said Phillip Gardiner of the African American Tobacco Control Leadership Council."Menthol cigarettes and flavored cigars are the main vectors of those diseases in the Black and brown communities, and have been for a long time.
Singapore Latest News, Singapore Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 FDA Announces Recall of Popular Blood Pressure Medication Due to Potential Cancer RiskIt’s time to double-check your medicine cabinets.
FDA Announces Recall of Popular Blood Pressure Medication Due to Potential Cancer RiskIt’s time to double-check your medicine cabinets.
Read more »
 FDA Approves First COVID Treatment for Use in KidsThe FDA has approved the antiviral remdesivir as the first COVID-19 treatment for young children.
FDA Approves First COVID Treatment for Use in KidsThe FDA has approved the antiviral remdesivir as the first COVID-19 treatment for young children.
Read more »
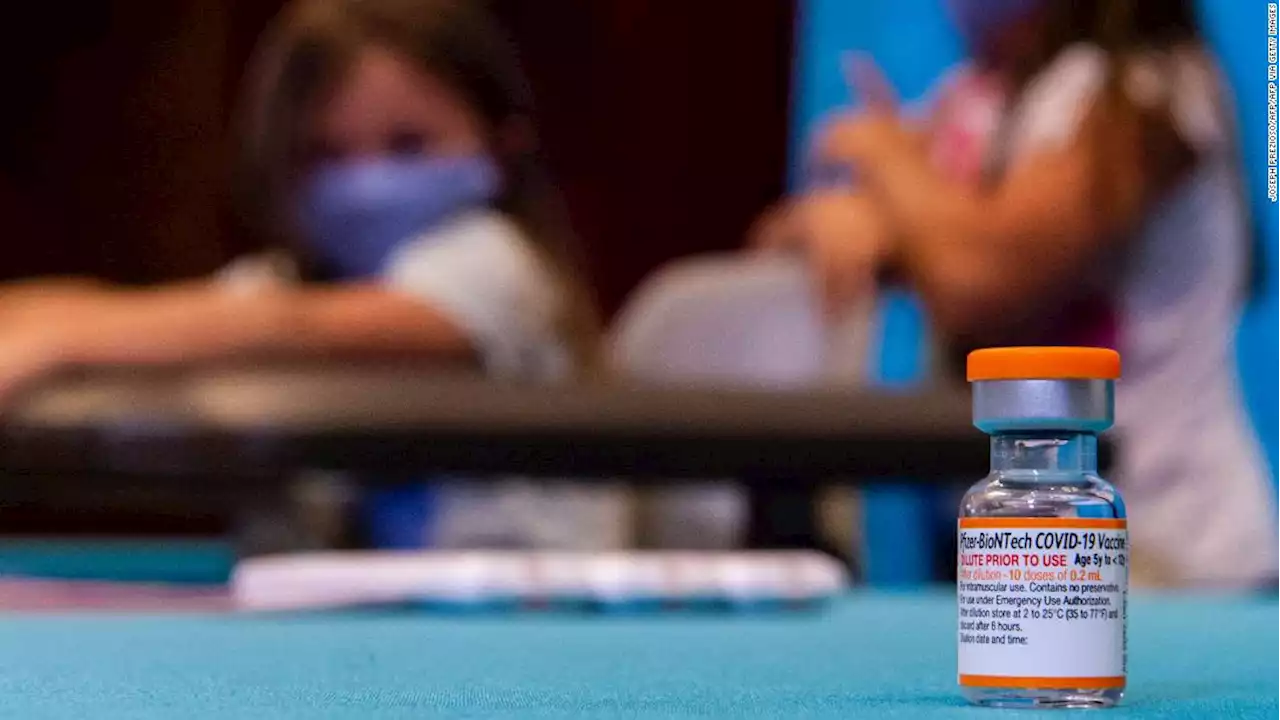 Pfizer requests FDA authorization for Covid-19 booster for kids 5 through 11Pfizer and BioNTech said Tuesday that they have submitted an application to the US Food and Drug Administration for emergency use authorization of a booster dose of Covid-19 vaccine for children 5 through 11.
Pfizer requests FDA authorization for Covid-19 booster for kids 5 through 11Pfizer and BioNTech said Tuesday that they have submitted an application to the US Food and Drug Administration for emergency use authorization of a booster dose of Covid-19 vaccine for children 5 through 11.
Read more »
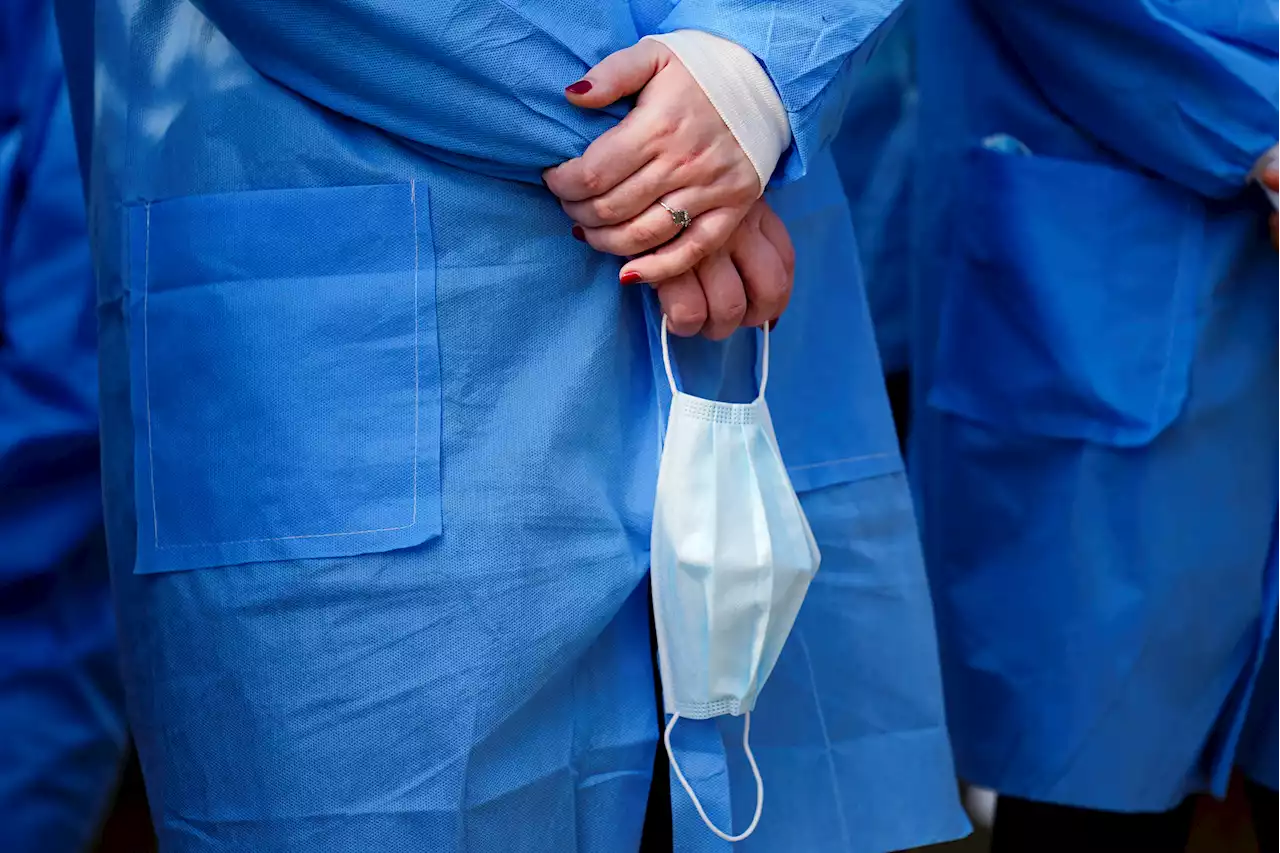 Pfizer Asks FDA to Authorize Third Dose for Children 5 to 11 Years OldPfizer on Tuesday asked the Food and Drug Administration to authorize a third dose of its COVID vaccine for children ages 5- to 11-years-old. The application comes after Pfizer released data earlier this month from a small lab study of blood samples from 30 kids in the age group, which showed a 36-fold increase in antibody levels against the omicron variant…
Pfizer Asks FDA to Authorize Third Dose for Children 5 to 11 Years OldPfizer on Tuesday asked the Food and Drug Administration to authorize a third dose of its COVID vaccine for children ages 5- to 11-years-old. The application comes after Pfizer released data earlier this month from a small lab study of blood samples from 30 kids in the age group, which showed a 36-fold increase in antibody levels against the omicron variant…
Read more »
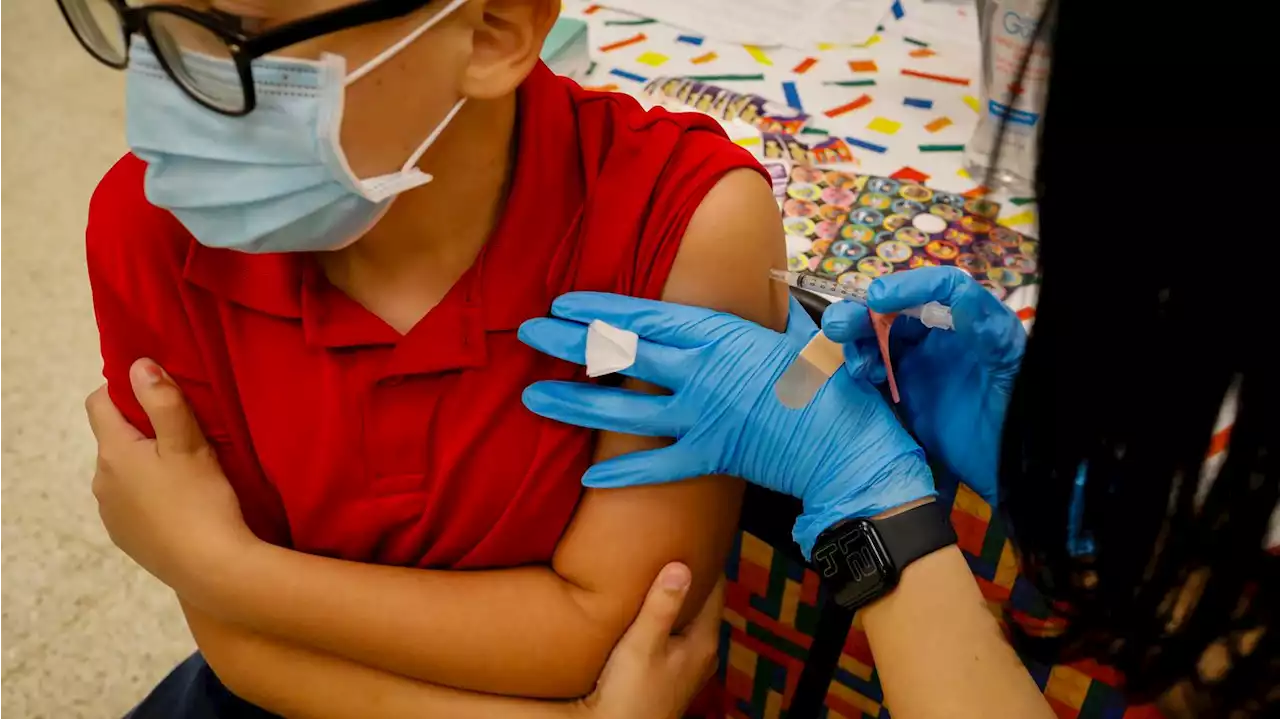 Pfizer asks FDA to authorize COVID booster for children 5 to 11If approved, it would be the first booster accessible to children of this age group, who were hit hard by Omicron.
Pfizer asks FDA to authorize COVID booster for children 5 to 11If approved, it would be the first booster accessible to children of this age group, who were hit hard by Omicron.
Read more »
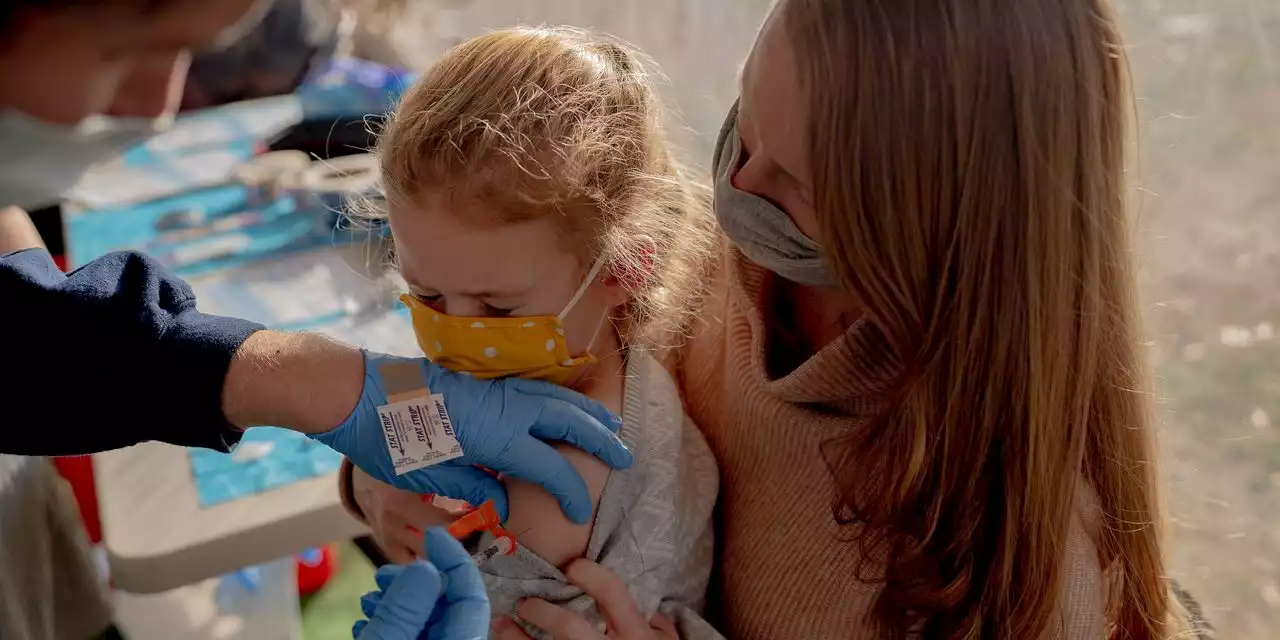 Pfizer Asks FDA to Authorize Covid-19 Booster for 5- to 11-Year-OldsPfizer Inc. and partner BioNTech SE said earlier this month that a third shot safely generated a strong immune response in the youngsters.
Pfizer Asks FDA to Authorize Covid-19 Booster for 5- to 11-Year-OldsPfizer Inc. and partner BioNTech SE said earlier this month that a third shot safely generated a strong immune response in the youngsters.
Read more »
