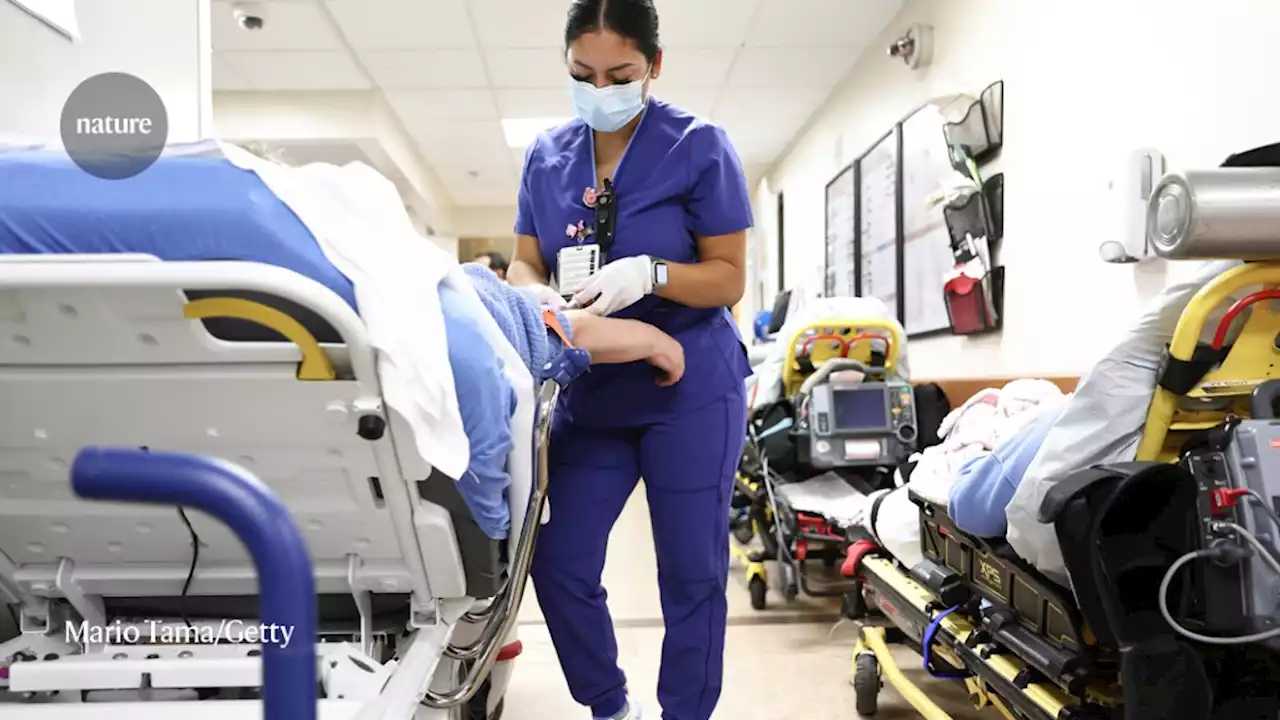
The world’s largest study of long COVID will evaluate potential therapies for brain fog, sleep disruption and more
Responding to this criticism, Kanecia Zimmerman, a clinician at Duke University School of Medicine in Durham, North Carolina, who is helping to coordinate the RECOVER studies, said at a press briefing yesterday that there are many steps to launching a clinical trial, including drafting study protocols, consulting with specialists and people with the disease and getting approval from authorities.
The first of the RECOVER treatment trials, which is already enrolling participants will evaluate Paxlovid, an antiviral made by New York City-based pharmaceticals firm Pfizer. A five-day regimen of Paxlovid pills is typically prescribed to people with acute COVID-19. Some research has found, though, that the virus can continue to cause symptoms and persist in the body for months after treatment.
The trial investigating brain fog, set to launch within weeks, will test two online training programmes and a home-based brain-stimulation device. “I’m encouraged by the comprehensiveness of the approach”, says Jim Jackson, a neuropsychologist at Vanderbilt University Medical Center in Nashville, Tennessee. If the therapies are proven safe and effective, they could be easily scaled and done at home, which would be a boon for accessibility, he adds.
Officials have delayed a fifth trial focused on the fatigue that people with long COVID experience after exercise. The protocols for that trial
Singapore Latest News, Singapore Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 Biden administration opens new office to study long COVID response, NIH begins clinical trialsThe Department of Health and Human Services announced a new office will be studying long COVID as the National institutes of Health beings clinical trials for treatments.
Biden administration opens new office to study long COVID response, NIH begins clinical trialsThe Department of Health and Human Services announced a new office will be studying long COVID as the National institutes of Health beings clinical trials for treatments.
Read more »
 Virus remains Biden focus as NIH launches long COVID trialsThe National Institutes of Health on Monday said it’s launching trials to test drugs and other treatments for long COVID, a mysterious condition that affects millions of people after they catch the coronavirus.
Virus remains Biden focus as NIH launches long COVID trialsThe National Institutes of Health on Monday said it’s launching trials to test drugs and other treatments for long COVID, a mysterious condition that affects millions of people after they catch the coronavirus.
Read more »
 US NIH launches long-COVID trials of Pfizer's Paxlovid, other therapiesThe U.S. National Institutes of Health (NIH) said on Monday that it had launched mid-stage clinical trials to test at least four treatments, including Pfizer's Paxlovid, in patients with symptoms of long COVID.
US NIH launches long-COVID trials of Pfizer's Paxlovid, other therapiesThe U.S. National Institutes of Health (NIH) said on Monday that it had launched mid-stage clinical trials to test at least four treatments, including Pfizer's Paxlovid, in patients with symptoms of long COVID.
Read more »
 First long COVID treatment clinical trials from NIH getting underwayResearchers will test a range of potential treatments, from melatonin to Paxlovid, to see if they relieve symptoms of long COVID.
First long COVID treatment clinical trials from NIH getting underwayResearchers will test a range of potential treatments, from melatonin to Paxlovid, to see if they relieve symptoms of long COVID.
Read more »
 NIH announces long covid treatment studies with hundreds of patientsThe National Institutes of Health announced Monday that it is enrolling hundreds of patients in four studies testing the safety and effectiveness of potential long-covid treatments, and expects to launch seven more clinical trials in the coming months.
NIH announces long covid treatment studies with hundreds of patientsThe National Institutes of Health announced Monday that it is enrolling hundreds of patients in four studies testing the safety and effectiveness of potential long-covid treatments, and expects to launch seven more clinical trials in the coming months.
Read more »