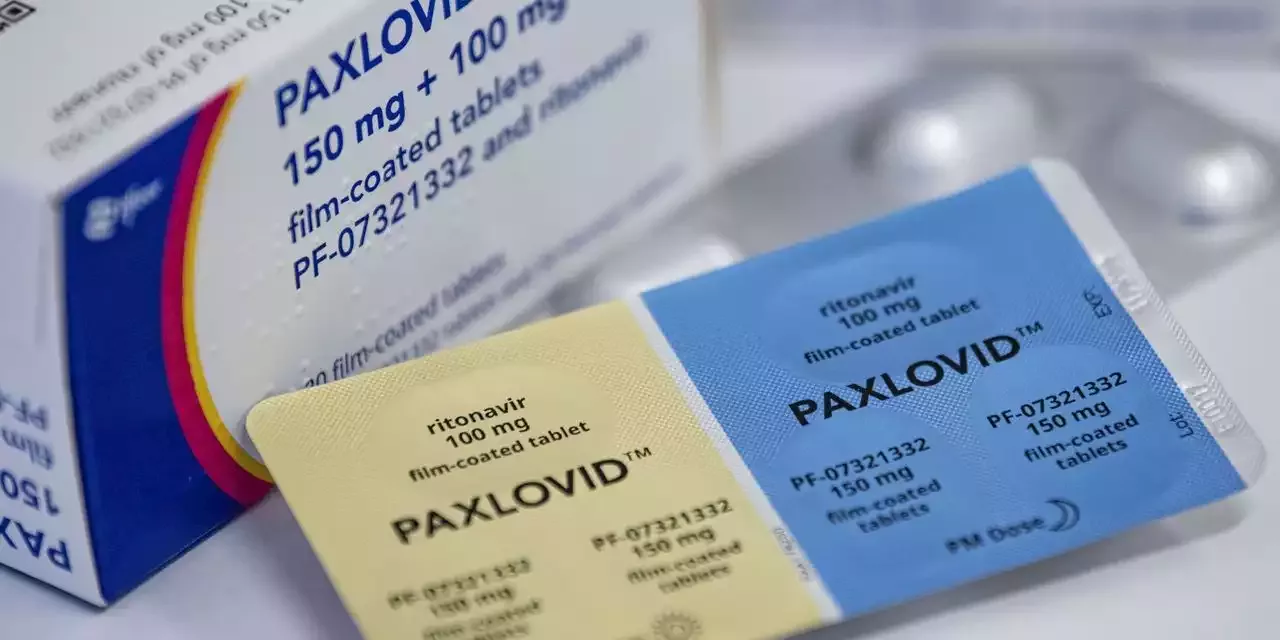
Pfizer’s Covid-19 pill Paxlovid wasn’t found to prevent symptomatic Covid-19 infections in a study. The pill is authorized to treat people at high risk of severe disease within a few days of infection
Paxlovid, authorized to treat high-risk people early in infection, didn’t meaningfully reduce illness in exposed adults
As fourth doses of Covid vaccines roll out, some are questioning whether the general population needs them. At the center of this debate are mysterious T-cells. WSJ’s Daniela Hernandez explains T-cells’ role in Covid immunity and how they relate to antibodies. Illustration: Laura KammermannThe Covid-19 pill from Pfizer Inc. failed to prevent symptomatic infections in adults who had been exposed to the pandemic virus, a late-stage study found.
Pfizer said Friday that the drug, named Paxlovid, failed the study’s main objective of meaningfully reducing the risk of confirmed and symptomatic
Singapore Latest News, Singapore Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 How Gilead is positioning Veklury against COVID-19 antivirals like PaxlovidThe company has an oral version of its COVID-19 drug Veklury in a clinical trial.
How Gilead is positioning Veklury against COVID-19 antivirals like PaxlovidThe company has an oral version of its COVID-19 drug Veklury in a clinical trial.
Read more »
 Covid-19: NI care home relatives call for answers on Covid testsFamilies ask why untested hospital patients were sent to care homes at the start of the pandemic.
Covid-19: NI care home relatives call for answers on Covid testsFamilies ask why untested hospital patients were sent to care homes at the start of the pandemic.
Read more »
 What is Paxlovid, Pfizer’s COVID antiviral drug, and how effective is it?It is the drug that has been hailed by the White House as key to the fight against COVID-19. Vice President Kamala Harris was prescribed it after she tested positive for the virus. We’re talking about Paxlovid, the antiviral medication from Pfizer.
What is Paxlovid, Pfizer’s COVID antiviral drug, and how effective is it?It is the drug that has been hailed by the White House as key to the fight against COVID-19. Vice President Kamala Harris was prescribed it after she tested positive for the virus. We’re talking about Paxlovid, the antiviral medication from Pfizer.
Read more »
 Should You Use Paxlovid If You Have Asymptomatic COVID?Here's what experts know about how well the antiviral works if you have little to no COVID symptoms.
Should You Use Paxlovid If You Have Asymptomatic COVID?Here's what experts know about how well the antiviral works if you have little to no COVID symptoms.
Read more »
 Moderna Asks FDA to Approve Its COVID-19 Shot for Kids Under 6Moderna on Thursday asked U.S. regulators to authorize low doses of its COVID-19 vaccine for children younger than 6, a long-awaited move toward potentially opening shots for millions of tots by summer.
Moderna Asks FDA to Approve Its COVID-19 Shot for Kids Under 6Moderna on Thursday asked U.S. regulators to authorize low doses of its COVID-19 vaccine for children younger than 6, a long-awaited move toward potentially opening shots for millions of tots by summer.
Read more »
 Shareholder Proposals to Broaden Access to Covid-19 Vaccines RejectedInvestors voted down shareholder proposals urging Pfizer, Moderna and Johnson & Johnson to broaden access to their Covid-19 vaccines
Shareholder Proposals to Broaden Access to Covid-19 Vaccines RejectedInvestors voted down shareholder proposals urging Pfizer, Moderna and Johnson & Johnson to broaden access to their Covid-19 vaccines
Read more »