Science, Space and Technology News 2023
The U.S. Food and Drug Administration has been approving a rising number of novel pharmaceutical drugs based on single clinical trials, with less public disclosure about these trials, according to two recent studies conducted by
“We’re not saying that cancer drugs need a lot more studies; just that they should show all the results or trials that are completed,” said Irvin, an associate professor in OSU’s College of Health. “It doesn’t mean they wouldn’t get approved, but it means we’d have a more complete picture.”, passed with bipartisan support in 2016 and meant to accelerate approval of new medicines so patients could gain access to life-saving drugs that would otherwise take years to become available.
In 2016, prior to the Cures Act, only four of 20 novel drugs were approved based on a single trial.article, researchers found that of the 46 novel drugs approved in 2017, 19 were approved based on results from a single study — though the drugmakers conducted an average of 2.2 studies per drug, including 165 studies for the popular weight-loss drug Ozempic.
Singapore Latest News, Singapore Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 FDA targeting 'candy-like' drugs that risk accidental overdose in kidsMatthew Perry seen dining with female pal, appeared in good spirits just 24 hours before death
FDA targeting 'candy-like' drugs that risk accidental overdose in kidsMatthew Perry seen dining with female pal, appeared in good spirits just 24 hours before death
Read more »
 FDA advisers see no roadblocks for gene-editing treatment for sickle cell diseaseAdvisers to the Food and Drug Administration meeting Tuesday paved the way for the first treatment of human disease using the gene-editing technique CRISPR. The agency has a December deadline.
FDA advisers see no roadblocks for gene-editing treatment for sickle cell diseaseAdvisers to the Food and Drug Administration meeting Tuesday paved the way for the first treatment of human disease using the gene-editing technique CRISPR. The agency has a December deadline.
Read more »
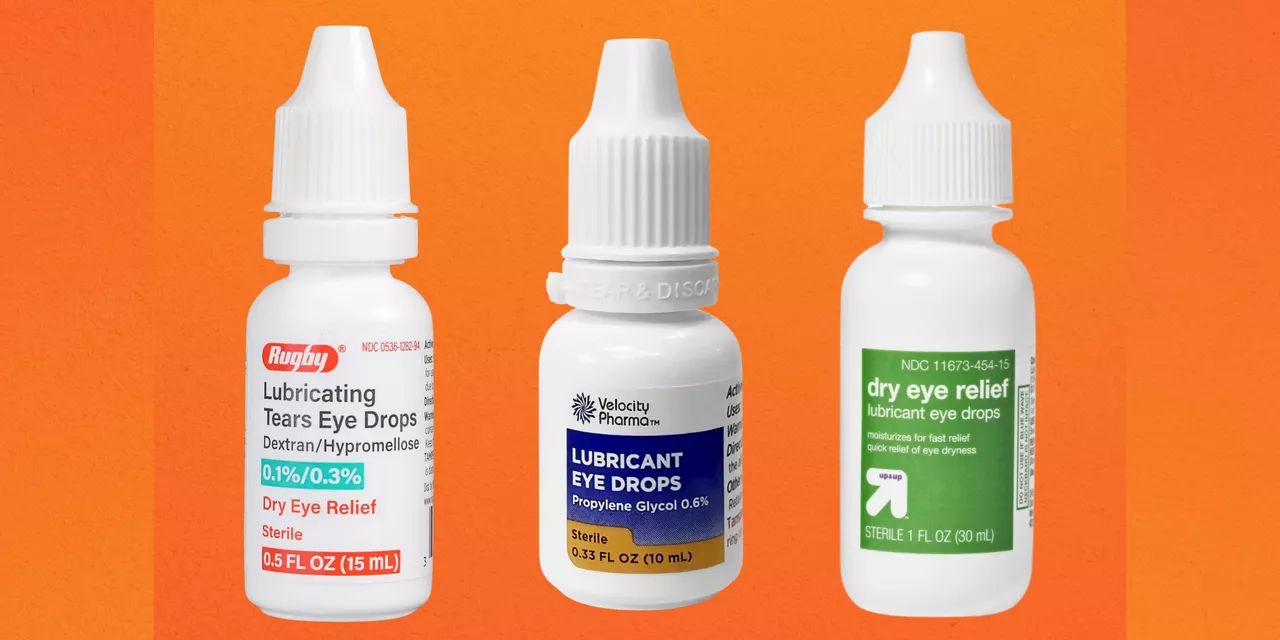 FDA Warns Against Using 26 Eye Drop Brands That Might Lead to InfectionThe FDA just issued a warning against 26 different brands of over-the-counter eye drops, including products sold at CVS Health and Rite Aid.
FDA Warns Against Using 26 Eye Drop Brands That Might Lead to InfectionThe FDA just issued a warning against 26 different brands of over-the-counter eye drops, including products sold at CVS Health and Rite Aid.
Read more »
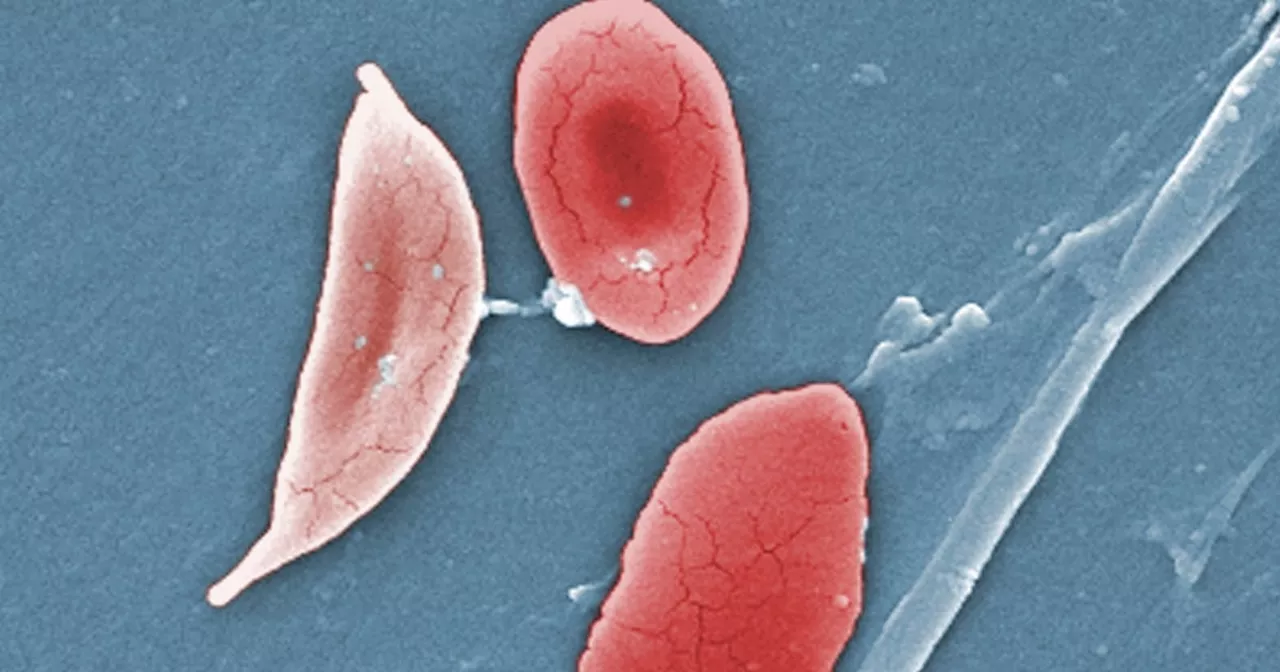 FDA moves closer to sickle cell cure that uses gene editingBerkeley Lovelace Jr. is a health and medical reporter for NBC News. He covers the Food and Drug Administration, with a special focus on Covid vaccines, prescription drug pricing and health care. He previously covered the biotech and pharmaceutical industry with CNBC.
FDA moves closer to sickle cell cure that uses gene editingBerkeley Lovelace Jr. is a health and medical reporter for NBC News. He covers the Food and Drug Administration, with a special focus on Covid vaccines, prescription drug pricing and health care. He previously covered the biotech and pharmaceutical industry with CNBC.
Read more »
 FDA Approves Secukinumab for Adults With Hidradenitis SuppurativaThis marks the second FDA-approved agent for the condition, and the first IL-17A inhibitor.
FDA Approves Secukinumab for Adults With Hidradenitis SuppurativaThis marks the second FDA-approved agent for the condition, and the first IL-17A inhibitor.
Read more »
 FDA panel says Vertex/CRISPR to assess safety risks of gene therapy in follow-up studyA panel of advisers to the U.S. health regulator said on Tuesday Vertex Pharmaceuticals (VRTX.O) and CRISPR Therapeutics (CRSP.BN) could assess potential safety risks of their sickle cell disease gene therapy after approval.
FDA panel says Vertex/CRISPR to assess safety risks of gene therapy in follow-up studyA panel of advisers to the U.S. health regulator said on Tuesday Vertex Pharmaceuticals (VRTX.O) and CRISPR Therapeutics (CRSP.BN) could assess potential safety risks of their sickle cell disease gene therapy after approval.
Read more »
